Calcium carbonate is an inorganic compound with the chemical formula CaCO₃. In nature, it takes on multiple forms, including calcite, limestone, marble, white marble, chalk, stalactites, and travertine, among others. It is also the main component of coral, shells, eggshells, and more.
1. Calcite

Calcite is the most common calcium carbonate mineral in nature. It is named “calcite” because many square fragments can be obtained by breaking it. Calcite is the main mineral that makes up limestone, marble, stalactites, and Iceland spar, among other minerals. The calcium carbonate content of calcite is usually higher than that of marble and limestone, reaching more than 99%.
Calcite can be divided into large calcite, small calcite, and Iceland spar. Large calcite has clear, regular cleavage and high transparency, while small calcite has disordered, fine, and irregular cleavage. Calcite itself is colorless or white but usually contains elements such as iron, manganese, and copper, giving it various colors.
Calcite is a rock-forming mineral on Earth, accounting for more than 40% of the total crust, and there are no fewer than 200 species of it.
2. Iceland Spar
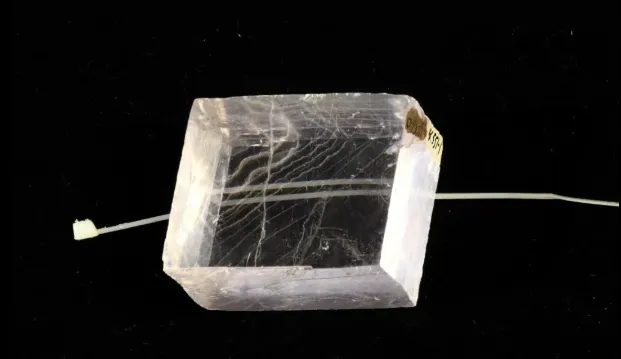
Iceland spar, a type of colorless and transparent calcite, is the purest calcium carbonate crystal found in nature. It was first discovered in Iceland, hence the name “Iceland spar.” Iceland spar has strong birefringence and the largest polarization function. It is an important optical material and a natural crystal that cannot be artificially manufactured or replaced.
High-quality Iceland spar crystals are produced in calcite veins and zeolite calcite veins in basalt. They are often used in polarizing prisms and polarizers in the optical industry. Iceland spar is also essential for making sunspot instruments and macrometers for astronomy, and is used in prisms for gem dichroic mirrors.
3. Limestone (Rock)

Limestone is the trade name for limestone as a mineral raw material. It is a carbonate rock, with calcite as the main component. It often contains dolomite, clay minerals, and detrital minerals, and it can be gray, grayish white, grayish black, yellow, light red, brownish red, and other colors.
Limestone is used as a raw material in the production of cement, glass, calcium carbide, soda ash, calcium oxide, calcium hydroxide, light calcium carbonate, and more. It can also be directly processed into stone and crushed stone for use as building materials. Limestone is widely used in many fields, such as metallurgy, environmental protection, food, medicine, rubber, plastics, papermaking, ink, coatings, cosmetics, and more.
4. Marble (Rock)

Marble is mainly composed of calcite and dolomite, with trace amounts of wollastonite, talc, tremolite, diopside, plagioclase, quartz, periclase, and other minerals. It has a granular metamorphic structure and a blocky (sometimes banded) structure. Pure marble is white, but when it contains impurities, it can present various colors and beautiful stripes. It is the main decorative building stone and carving stone.
The largest reserves of white marble in China are located in Baoxing, Sichuan, and Hezhou, Guangxi. The calcium carbonate content of Hezhou white marble is greater than 99%, with a whiteness greater than 95%. It is known for its high whiteness, good purity, soft hue, stable chemical composition, and the absence of sulfides and other elements harmful to the human body. This marble is well-known both domestically and internationally.
The white marble from Baoxing, Sichuan, has finer particles, purer whiteness, and better quality. It is considered one of the finest products in the current development of white marble in China.
5. White Marble

White marble is a high-quality variety of white marbles. It is mainly composed of CaCO₃, MgCO₃, and SiO₂, and also contains small amounts of Al₂O₃, Fe₂O₃, and other elements.
The genuine white marble is Beijing Fangshan White, which is produced in Gaozhuang Village, Dashiwo Town, Fangshan District, southwest of Beijing. The reserve is 800,000 cubic meters, making it the largest in the country. Fangshan White is considered a “national treasure” and is famous for its fine, hard texture, white and delicate quality, and its solemn, rich style. It is as white as snow, as hard as jade, frosty, clear, and elegant, giving it a majestic and solemn appearance. It has become a symbol of auspiciousness, elegance, and wealth, and has long been a royal product of various dynasties, also known as “White Imperial Stone.”
6. Chalk

Chalk is a fine calcium carbonate deposit, a variant of calcite. Its name comes from the Latin word for “clay,” and it refers to the common chalk found in the Upper Cretaceous strata, which is formed by the calcium carbonate deposits of marine invertebrate shells, especially coccoliths.
Chalk is soft, loose, and porous, and is often used as a plastering material. For example, early chalk was used in the creation of chalk sculptures. In Europe, about 16% of heavy calcium carbonate is produced from chalk.
7. Stalactites

Also known as stalactites, this term refers to the general classification of calcium carbonate deposits, such as stalactites, stalagmites, and stone columns, which form in caves in carbonate rock areas under specific geological conditions over a long period of time.
The main component of limestone is calcium carbonate. When it reacts with water containing carbon dioxide, calcium bicarbonate, which is more soluble, is formed. When water containing calcium bicarbonate is heated or the pressure decreases suddenly, the calcium bicarbonate dissolved in the water decomposes and regenerates calcium carbonate, which then precipitates and releases carbon dioxide. When water seeps slowly from the top of a cave, some of the calcium bicarbonate in the water undergoes the same reaction. Some of it is deposited on the top of the cave, and some is deposited on the bottom. Over time, stalactites form on the top, and stalagmites form on the bottom. When stalactites connect with stalagmites, stone columns are formed.
8. Travertine

Travertine, also known as travertine limestone, is a chemical precipitate of calcium carbonate that forms when carbon dioxide escapes from groundwater containing calcium bicarbonate as it reaches the surface. It is a macroporous secondary calcium carbonate formed by the deposition of karst springs, rivers, and lakes. The mineral components of travertine are primarily calcite and aragonite.
Travertine has a varied morphology, with common formations including travertine cones, hills, and fans.
It is widely distributed in terrestrial environments, especially in limestone areas with high precipitation and high annual average temperatures. The Huanglong National Scenic Area, located in Songpan County, Sichuan Province, is home to a rare plateau cold-water travertine landscape. It was listed as a UNESCO World Natural Heritage site in 1992 and is known for having the world’s three best features: the most spectacular open-air calcified pools, the largest travertine beach flow, and the largest travertine collapse wall.
9. Coral

Coral is formed by the aggregation of calcareous skeletons secreted by coral polyps. Its chemical composition is primarily CaCO₃, which exists in the form of microcrystalline calcite aggregates, with a certain amount of organic matter also present. Corals are typically tree-like in shape, with vertical stripes, and are mostly white in color, though they may also appear in shades of blue and black.
10. Shell

A shell is the mantle of mollusks that live near water. It is a calcified substance formed by the secretion of a special glandular cell in the mollusk to protect the soft part of its body.
Shells are mainly composed of two phases: an inorganic phase and an organic phase. The inorganic phase consists of 95-99.9% CaCO₃ (in the forms of calcite, aragonite, vaterite, and amorphous forms). Under standard room temperature conditions, calcite is the most stable form, aragonite is relatively stable, and vaterite is the most unstable. The organic phase makes up about 0.1-5% of the shell’s composition and includes proteins, glycoproteins, polysaccharides, chitin, lipids, and other elements such as calcium, carbon, oxygen, hydrogen, strontium, and magnesium.
Compared to natural calcium carbonate minerals, shells have a unique multi-scale, multi-level “brick-and-mortar” assembly structure. This structure provides them with excellent properties, such as high toughness and strength.
Studies have shown that active calcium oxide prepared from scallop shells has better activity and stronger antibacterial effects than ordinary calcium oxide. Additionally, the bioactive material hydroxyapatite, which was prepared from discarded shells using a water bath heating method, showed excellent in vitro biological activity. By using shell powder as the raw material, diammonium hydrogen phosphate as the phosphorus source, and urea as the additive, high-purity, uniform, and controllable nano-belt hydroxyapatite was successfully produced. Furthermore, when shells were soaked in a phosphate buffer for 24 hours, a layer of carbonate-type apatite with a flaky structure was deposited on the shell surface through a dissolution-recrystallization reaction. The results indicated that the prepared apatite exhibited excellent biological activity, highlighting the potential of shells and their components for research in biomaterials and bioactive substances.
11. Eggshell
Eggshells are composed of 93% CaCO₃, 1.0% MgCO₃, 2.8% Mg₃(PO₄)₂, and 3.2% organic matter.
Waste eggshells contain rich calcium resources and are an important raw material for bio-based calcium carbonate and calcium oxide. They are considered safe and environmentally friendly and can be used as catalysts, adsorbents, additives, and more. Eggshell calcium carbonate can be used to prepare a variety of calcium-based products, such as calcium acetate, calcium propionate, calcium lactate, calcium gluconate, calcium citrate, calcium pyruvate, calcium malate, juice calcium, calcium sulfate, and calcium peroxide.
About Epic Powder
Epic Powder Machinery specializes in the development and manufacturing of jet mills and powder processing solutions. Contact us today for a free consultation and customized solutions! Our expert team is dedicated to providing high-quality products and services to maximize the value of your powder processing. Epic Powder—Your Trusted Powder Processing Expert!